An article published in the journal “Cell” describes the experimentation on mice of a variant of the CRISPR/Cas9 genetic modification system to treat a number of diseases. A team of researchers from the Salk Institute for Biological Studies used a version of CRISPR/Cas9 that doesn’t modify the DNA avoiding the risk of causing unexpected mutations but can activate one or more genes creating an epigenetic therapy for diseases such as Type 1 diabetes, acute kidney disease or muscular dystrophy.
The CRISPR (clustered regularly interspaced short palindromic repeats) acronym refers to prokaryotic DNA segments containing short repeated sequences. The expression CRISPR/Cas refers to a prokaryotic immune system conferring a genetic resistance to foreign elements. The CRISPR/Cas system is used in genetic engineering, in particular using the Cas9 (CRISPR associated protein 9) enzyme, that evolved in bacteria of the species Streptococcus pyogenes as part of their immune system.
One of the problems yet to be assessed with precision is that a DNA modification carried out with one of the various CRISPR systems being tested might cause a damage to the genome and as a consequence an unexpected genetic mutation. In order to avoid this risk, in recent years some experiments have been carried out of a different use of the CRISPR systems at the epigenetic level, meaning not modifying an organism’s DNA but activating some specific genes useful to treat a disease.
This result was obtained for the first time not in vitro but in experiments with mice using a variant of the CRISPR/Cas9 system, a “dead” Cas9 called dCas9 that can aim to a specific target within an organism’s DNA but is no longer able to cut it. Instead, it was paired with a molecular switch that can activate the gene targeted by dCas9.
The CRISPR systems use viruses to be transported in cells but dCas9 is very large from a genetic point of view so the researchers split it into two parts: one that contains the “guide” system and one that contains the molecular switches together with the RNA that targets the genes to be activated. These two parts are transported by two adeno-associated viruses (AAVs), viruses that can’t infect cells but can carry CRISPR/dCas9.
The experiments concerned various diseases for which an epigenetic therapy can be created. In the case of acute kidney disease, they activated two genes connected to kidney’s functions. In the case of type 1 diabetes, they stimulated the activity of genes that can generate insulin-producing cells. In the case of muscular dystrophy, they activated genes known for their ability to reverse the disease’s symptoms.
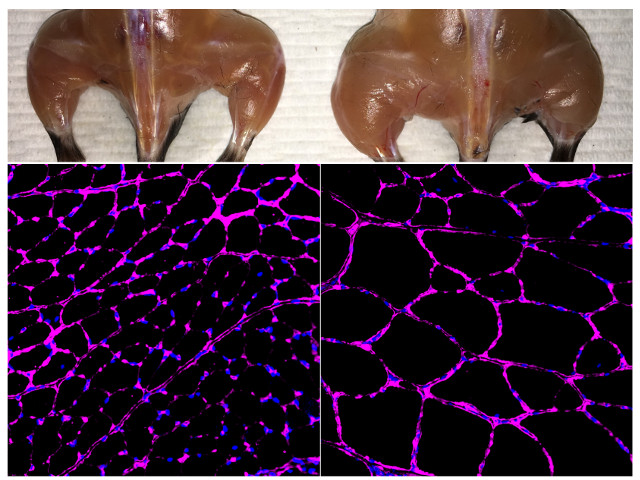
The image (courtesy Salk Institute) shows some results of the experiments on mice. On the left, tissues of an untreated mouse are shown, on the right those of a mouse treated with the CRISPR/dCas9 system. At the top the image shows how the activation of certain genes enhanced skeletal muscle mass, at the bottom how it stimulates fiber size growth. The images under the fluorescent microscope at bottom shows a purple staining of the laminin glycoprotein in tibial anterior muscle fibers.
The results are encouraging but there’s still a lot of work to be done before moving to human applications. The next tests will be carried out on larger animal species to assess the different responses to treatments of different diseases. Future applications could also concern the rejuvenation of certain organs and reverting the degeneration of certain tissues.

