An article published in the journal “Nature” reports the use of cryo-electron microscopy to capture images of a genetic modification tool called INTEGRATE in the course of its work. A team led by Sam Sternberg of Columbia University applied this technique that uses a transposon to improve the results of a CRISPR-Cas system thanks to its ability to reliably insert DNA sequences into a genome. In essence, the use of a transposon eliminates the potential errors that CRISPR-Cas systems can create by cutting DNA.
An article published in the journal “Nature” reports the use of cryo-electron microscopy to capture images of a genetic modification tool called INTEGRATE in the course of its work. A team led by Sam Sternberg of Columbia University applied this technique that uses a transposon to improve the results of a CRISPR-Cas system thanks to its ability to reliably insert DNA sequences into a genome. In essence, the use of a transposon eliminates the potential errors that CRISPR-Cas systems can create by cutting DNA.
The CRISPR (clustered regularly interspaced short palindromic repeats) acronym refers to prokaryotic DNA segments containing short repeated sequences. The expression CRISPR/Cas refers to a prokaryotic immune system conferring a genetic resistance to foreign elements.
One of the problems of the various CRISPR systems is the risk that the DNA repair that follows the insertion of the new sequence after a cut is not perfect and generates errors that can have unpredictable consequences. Various advances have been made to limit this problem using different approaches, in this case a team of researchers reported in a article published in “Nature” in June 2019 a possible remedy based on the use of a transposon, a so-called jumping gene that can insert a genetic sequence more precisely. Now, a team almost identical to the one that described the INTEGRATE tool reports the results of some tests in terms of images.
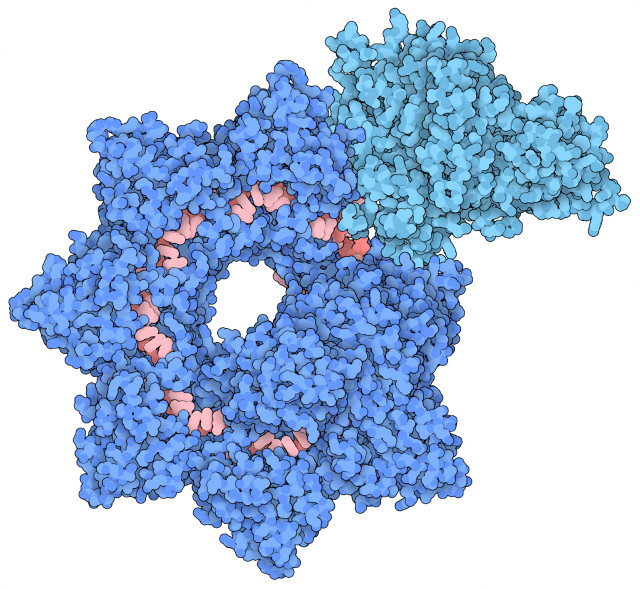
The top image (Courtesy Sternberg and Fernández Labs at Columbia University Irving Medical Center. All rights reserved) shows some images of the INTEGRATE complex captured using cryo-electron microscopy. The bottom image (Courtesy Sternberg and Fernández Labs/Columbia University Irving Medical Center. All rights reserved) shows the molecular structure of the INTEGRATE complex: in dark blue the structure called Cascade, in light blue the protein units of the transposon called TniQ and in red the RNA guide.
The Cascade structure carries an RNA guide that it uses to search for a certain DNA sequence. When it’s located, it uses the proteins called TniQ of the transposons found in bacteria of the Vibrio cholerae species to obtain a genetic modification more reliably than the CRISPR systems now in use such as CRISPR-Cas9 because it doesn’t have to rely on cellular repair mechanisms that carry the risk of generating errors.
Once again, scientists searched among the tools developed in nature to find the most suitable one to develop reliable systems to make genetic modifications. The INTEGRATE tool should also be better than the others when inserting long genetic sequences. There’s still a need for experiments before adopting this instrument in applications for medical purposes on humna being but the prospects are certainly interesting.